
Home > FAQ 6


If ammonia is used as fuel in thermal power generation, could it lead to an increase in emissions of substances that can be sources of PM2.5 in the entire supply chain (production, transportation, combustion)?
A. Technically and legally the use of ammonia as fuel in thermal power generation will not increase the emission of ammonia, nitrogen oxides, and other substances that can be the source of PM2.5 into the atmosphere, therefore PM2.5 should not increase.


To begin with: How ammonia affects PM2.5 formation
Ammonia (NH3) does not directly correspond to PM2.5 but has a secondary effect through the reaction of sulfur oxides (SOx) and nitrogen oxides (NOx) with ammonia (NH3) in the atmosphere, resulting in the formation of fine particulate matter (PM2.5) called sulfate and nitrate1).
- Sulfur dioxide (SO2) emitted into the atmosphere is oxidized as a gas or dissolved in water to form sulfuric acid (H2SO4). Sulfuric acid is stable as droplets in air, but reacts with ammonia (NH3) and other substances to form solid particles to produce sulfate.
- Nitric oxide (NO) produced during combustion is gradually oxidized in the atmosphere and reacts with water to form nitric acid (HNO3). Nitric acid exists as a gas by itself without becoming particulate due to its high saturated vapor pressure, but reacts with ammonia (NH3) and other substances to form nitrate.
Based on the above mechanism of how ammonia affects the formation of PM2.5, if the amount of SOx, NOx, and NH3 emitted during the production, transportation, and combustion of ammonia is kept at the same level or lower than the current situation where ammonia is not used, it can be said that PM2.5 will not increase by using ammonia as fuel in thermal power plants. The following paragraphs provide details for each part of production, transportation, and combustion.
SOx, NOx, and NH3 emissions during “PRODUCTION”
- SOx and NOx: Ammonia production plants are designed and constructed after obtaining strict air pollution control permits and approvals set by the country of construction. For example, according to the guidelines of the World Bank-affiliated IFC, SOx emissions should be less than 20 μg/m32) and NOx emissions less than 300 mg/Nm3).
- NH3: Ammonia production plants are strictly controlled to prevent leakage during operation, but there are cases of unavoidable gas emissions called fugitive emissions from valves and flanges. However, this amount is only about 0.001% of the total emissions4).
SOx, NOx, and NH3 emissions during “TRANSPORTATION”
- SOx and NOx: Ship fuel is also being considered for conversion to ammonia, but this will not lead to an increase in SOx and NOx emissions because international regulations for SOx and NOx emissions from ships are strictly set and standards must be met. Ammonia does not contain sulfur, which causes SOx. As for NOx, development is underway in Japan and abroad for both improvements to optimize the reduction of NOx emissions in internal combustion engines and the removal of NOx, including residual NH3 and N2O in the exhaust gas, using catalytic cracking equipment5).
- NH3: Natural heat input evaporates NH3 in tanks, etc., to form boil off gas, which is re-liquefied by compressors, etc. and is not released into the atmosphere. Fugitive emissions are also much lower than in production because ships have fewer valves and flanges than plants.
SOx, NOx, and NH3 emissions during “COMBUSTION”
- SOx and NOx: Japanese thermal power plants are working to reduce emissions by installing flue gas desulfurization and denitrification equipment and improving combustion methods, and their emission intensity in FY2021 is extremely lower than that of major Western countries (0.03 g/kWh for SOx and 0.07 g/kWh for NOx)6).
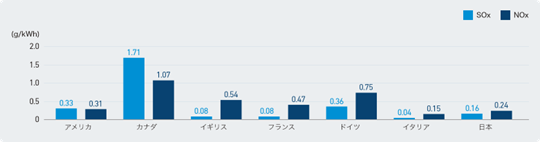
- NH3: It is true that ammonia is less flammable than conventional fossil fuels, but by using appropriate combustion methods, it is possible to reduce unburned NH3 to almost zero (less than 1 ppm at 20% co-firing7). Even if there is some residual combustion, NH3 is consumed in the flue gas denitrification equipment already in use at thermal power plants, and the amount of unreacted NH3 at the outlet of the denitrification equipment is strictly controlled to be kept to a minimum8). Furthermore, the concentration of ammonia in the stack and at the site boundary is regulated by the Offensive Odor Control Law, and any increase in emissions is not allowed9).
- International Institute for Environmental Economics, Non-Profit Organization, “What’s PM2.5 Made of?” (October 28, 2013) (In Japanese)【Link】
- International Finance Corporation ’Environmental, Health, and Safety General Guidelines’【Link】
- International Finance Corporation ‘Environmental, Health, and Safety Guidelines for Nitrogenous Fertilizer Production’【Link】
- United States Environmental Protection Agency ‘Protocol for Equipment Leak Emission Estimates’【Link】
- International Shipping GHG Zero Emission Project “Toward Achieving Carbon Neutrality in International Shipping by 2050” (March 2022) (In Japanese)【Link】
- JERA Website ‘Environment’【Link】
- IHI Engineering Review Vol.55, No.2 (2022) ‘Development of Co-Firing Technology of Pulverized Coal and Ammonia for Suppressing NOx Generation’【Link】
- International Institute for Environmental Economics Environmental Technology Explanation “Flue Gas Denitration Technology (In Japanese)【Link】
- Ministry of Environment ‘The Offensive Odor Control Law in Japan’【Link】

9F Takebashi Bldg., 2-1-8 Kandanishikicho, Chiyoda-ku, Tokyo 101-0054, Japan
EMAIL : office@greenammonia.org
